Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Mizuno, Takashi; Milodowski, A. E.*; Iwatsuki, Teruki
Chemical Geology, 603, p.120880_1 - 120880_16, 2022/08
Times Cited Count:3 Percentile:56.2(Geochemistry & Geophysics)This study has focused on the formation sequence and Rare earth elements with yttrium (REY) of fracture-filling calcite in the Toki Granite in the Mizunami area, central Japan. The morphological, chemical and isotopic characteristics of the calcite and chemistry of fluid inclusions reveal that the calcite in the Toki Granite can be differentiated into four discrete generations: Calcite I (oldest) to Calcite IV (most recent). The precipitation history of calcite reflects the changes in the hydrogeochemical regime of paleo-groundwaters, controlled by the evolution of groundwater by seawater infiltration associated with marine transgression and surface water infiltration associated with marine regression and uplift. The post-Archean average shale-normalized REY patterns in generations of calcite show no significant Ce anomaly, negative Eu anomaly, and light REY (LREY)-depleted pattern in dominates. These features are also common to the Toki Granite. The consistency of the features in each generation of calcite indicates that REY was supplied from the Toki Granite by water-rock interaction. The lack of a Ce anomaly in the calcite demonstrates that groundwaters have maintained reducing conditions during the calcite precipitation. However, the fractionation of LREY and heavy REY (HREY) in each generation of the calcite is more pronounced than in the granite. The fractionation process in the paleo-groundwaters from which each generation of calcite precipitated closely relates to the systematic variation of carbonate complex in REY series and/or pH in palaeo-groundwater. The findings of this study will be necessary for assessing the long-term safety of geological disposal of high-level radioactive waste.
Yu, Q.*; Onuki, Toshihiko*; Kozai, Naofumi; Sakamoto, Fuminori; Tanaka, Kazuya; Sasaki, Keiko*
Chemical Geology, 470, p.141 - 151, 2017/10
Times Cited Count:15 Percentile:55.72(Geochemistry & Geophysics)In this work, the Cs retention onto two types of Mn oxide was investigated. We found that Todorokite has sorption sites with a higher selectivity for Cs than birnessite. When the initial Cs concentration was 10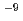 mol/L for the sorption experiments, approximately 34% of the sorbed Cs was residual in the todorokite after the extraction using 1 M NaCl and NH
mol/L for the sorption experiments, approximately 34% of the sorbed Cs was residual in the todorokite after the extraction using 1 M NaCl and NH Cl; this value was much higher than the results for the Cs-sorbed birnessite. These results strongly suggest that todorokite contributes to the fixation of radioactive Cs in soils.
Cl; this value was much higher than the results for the Cs-sorbed birnessite. These results strongly suggest that todorokite contributes to the fixation of radioactive Cs in soils.
 -MnO
-MnO in NaCl solution
in NaCl solutionTanaka, Kazuya; Tanaka, Masato*; Watanabe, Naoko*; Tokunaga, Kohei*; Takahashi, Yoshio*
Chemical Geology, 460, p.130 - 137, 2017/06
Times Cited Count:8 Percentile:34.27(Geochemistry & Geophysics)Pd is highly accumulated in ferromanganese nodules and crusts relative to its concentration in seawater but the mechanism by which Pd(II) is incorporated remains poorly understood. We investigated the local coordination structure of Pd(II) adsorbed on  -MnO
-MnO , using X-ray absorption fine structure spectroscopy. X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) analyses indicated that Pd was adsorbed on
, using X-ray absorption fine structure spectroscopy. X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) analyses indicated that Pd was adsorbed on  -MnO
-MnO through ligand exchange, from Cl coordination to O coordination. Furthermore, curve fitting of EXAFS spectra demonstrated the formation of two different inner-sphere complexes, bidentate-mononuclear and bidentate-binuclear complexes, and this finding was supported by density functional theory calculations. The formation of inner-sphere complexes is reasonable given the relatively large distribution coefficients obtained from adsorption experiments.
through ligand exchange, from Cl coordination to O coordination. Furthermore, curve fitting of EXAFS spectra demonstrated the formation of two different inner-sphere complexes, bidentate-mononuclear and bidentate-binuclear complexes, and this finding was supported by density functional theory calculations. The formation of inner-sphere complexes is reasonable given the relatively large distribution coefficients obtained from adsorption experiments.
Munemoto, Takashi; Omori, Kazuaki*; Iwatsuki, Teruki
Chemical Geology, 417, p.58 - 67, 2015/12
Times Cited Count:32 Percentile:72.97(Geochemistry & Geophysics)Rare earth elements (REEs) combined with yttrium (YREE) in deep groundwater from granite and fracture-filling calcite are being studied at the Mizunami Underground Research Laboratory (MIU, Tono area, central Japan).
 nanoparticles on microbial metabolism
nanoparticles on microbial metabolismMasaki, Shota*; Shiotsu, Hiroyuki; Onuki, Toshihiko; Sakamoto, Fuminori; Utsunomiya, Satoshi*
Chemical Geology, 391, p.33 - 41, 2015/01
Times Cited Count:9 Percentile:31.6(Geochemistry & Geophysics)Distinct organic species and intracellular proteins were expressed after exposure of yeast cells to CeNPs. Although cytotoxicity was not caused by CeNPs, the results of the peptide mass fingerprint analysis of the intracellular protein revealed that Eno2p, a glycolysis enzyme, was expressed after the exposure to CeNPs. These results suggest that nanoparticles have the potential to alter microbial metabolism, leading to changes in the compositions of the released substances in the surrounding environment.
Yamasaki, Seiko*; Zwingmann, H.*; Yamada, Kunimi*; Tagami, Takahiro*; Umeda, Koji
Chemical Geology, 351, p.168 - 174, 2013/08
Times Cited Count:25 Percentile:60.27(Geochemistry & Geophysics)Constraining of the timing of fault zone formation is of fundamental geotectonic importance to understand structural evolution and brittle fault processes. Here, we present authigenic illite K-Ar age data from brittle fault zones comprising two gouges within the Toki granite, central Japan. The gouge samples were collected from a shaft at the Mizunami Underground Research Laboratory, and were separated into five grain-size fractions. K-Ar ages of clay fractions decrease with grain size, suggesting enrichment in finer fraction of more-recently grown authigenic illites. The K-Ar ages of the fractions range from 53.6 to 42.7 Ma (Paleogene-Early to Middle Eocene). The  0.1
0.1  m fractions yield ages of 42.7 and 46.5 Ma. This age range is consistent with the stability field of illite and the main temperature field of brittle deformation within the cooling history of the Toki granite, supported by extensive thermochronological data of the host rock.
m fractions yield ages of 42.7 and 46.5 Ma. This age range is consistent with the stability field of illite and the main temperature field of brittle deformation within the cooling history of the Toki granite, supported by extensive thermochronological data of the host rock.
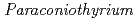 sp. WL-2 strain affecting sorption of Co
sp. WL-2 strain affecting sorption of Co
Yu, Q.*; Sasaki, Keiko*; Tanaka, Kazuya*; Onuki, Toshihiko; Hirajima, Tsuyoshi*
Chemical Geology, 310-311, p.106 - 113, 2012/06
Times Cited Count:63 Percentile:84.96(Geochemistry & Geophysics)These results strongly suggested that the interlayer Mn(III) can oxidize the adsorbed Co to Co
to Co , resulting in specific adsorption of Co
, resulting in specific adsorption of Co by biogenic birnessite.
by biogenic birnessite.
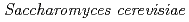
Jiang, M.; Onuki, Toshihiko; Kozai, Naofumi; Tanaka, Kazuya; Suzuki, Yoshinori*; Sakamoto, Fuminori; Kamiishi, Eigo*; Utsunomiya, Satoshi*
Chemical Geology, 277(1-2), p.61 - 69, 2010/10
Times Cited Count:37 Percentile:66.93(Geochemistry & Geophysics)We have investigated the mechanism underlying Ce sequestration by yeast 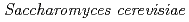 after exposure to Ce(III) solution at pH 3, 4, or 5. We found that needle-shaped Ce(III) phosphate nanocrystallites with a monazite structure formed on the yeast cells by exposure to Ce(III) for 42 h, even though the initial solutions did not contain any P species. These results suggest that the sorbed Ce on the cell surfaces reacted with P released from inside the yeast cell, resulting in the formation of Ce(III) phosphate nanocrystallites.
after exposure to Ce(III) solution at pH 3, 4, or 5. We found that needle-shaped Ce(III) phosphate nanocrystallites with a monazite structure formed on the yeast cells by exposure to Ce(III) for 42 h, even though the initial solutions did not contain any P species. These results suggest that the sorbed Ce on the cell surfaces reacted with P released from inside the yeast cell, resulting in the formation of Ce(III) phosphate nanocrystallites.
Onuki, Toshihiko; Ozaki, Takuo; Kozai, Naofumi; Nankawa, Takuya; Sakamoto, Fuminori; Sakai, Takuro; Suzuki, Yoshinori; Francis, A. J.*
Chemical Geology, 253(1-2), p.23 - 29, 2008/07
Times Cited Count:28 Percentile:55.18(Geochemistry & Geophysics)We examined the changes in the chemical states of Ce(III) during the formation of manganese oxide occasioned by Mn(II)-oxidizing bacteria. The oxidation states of Ce and Mn then were measured by X-ray Absorption Near Edge Structure (XANES). We also determined the elemental distributions in the bacteria and precipitates by Scanning-Proton Induced X-ray Emission (S-PIXE). We found that the precipitation of Ce is preceded by its accumulation by the bacterium, followed by its oxidization to Ce(IV) by the Mn(III, IV)-containing precipitates that the bacteria generate.
Murakami, Takashi*; Sato, Tsutomu*; Onuki, Toshihiko; Isobe, Hiroshi*
Chemical Geology, 221(1-2), p.117 - 126, 2005/09
Times Cited Count:56 Percentile:72.63(Geochemistry & Geophysics)High-resolution transmission and scanning electron microscopies (HRTEM and SEM) both equipped with energy dispersive X-ray spectrometers (EDS) have been used to determine the chemical form of U(VI) in rocks and to examine U uptake mechanisms at low U concentrations (10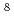
 10
10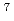 mol/L) in the downgradient of the Koongarra U ore deposit in Australia. We found that uranyl-phosphate nanocrystallization, though initiated by adsorption, is a dominating mechanism of the U uptake and controls long-term U transport at low U concentrations.
mol/L) in the downgradient of the Koongarra U ore deposit in Australia. We found that uranyl-phosphate nanocrystallization, though initiated by adsorption, is a dominating mechanism of the U uptake and controls long-term U transport at low U concentrations.
Onuki, Toshihiko; Yoshida, Takahiro*; Ozaki, Takuo; Samadfam, M.*; Kozai, Naofumi; Yubuta, Kunio*; Mitsugashira, Toshiaki*; Kasama, Takeshi*; Francis, A. J.*
Chemical Geology, 220(3-4), p.237 - 243, 2005/08
Times Cited Count:52 Percentile:70.55(Geochemistry & Geophysics)no abstracts in English
Onuki, Toshihiko; Sakamoto, Fuminori; Kozai, Naofumi; Ozaki, Takuo; Yoshida, Takahiro; Narumi, Issei; Wakai, Eiichi; Sakai, Takuro; Francis, A. J.*
Chemical Geology, 212(3-4), p.279 - 290, 2004/12
Times Cited Count:17 Percentile:34.28(Geochemistry & Geophysics)no abstracts in English
 -Al
-Al O
O and
and 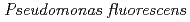 cells in the presence of desferrioxamine B; Implication of siderophores for the Ce anomaly
cells in the presence of desferrioxamine B; Implication of siderophores for the Ce anomalyYoshida, Takahiro; Ozaki, Takuo; Onuki, Toshihiko; Francis, A. J.*
Chemical Geology, 212(3-4), p.239 - 246, 2004/12
Times Cited Count:45 Percentile:63.36(Geochemistry & Geophysics)no abstracts in English
 )
) (PO
(PO )
) .
. H
H O) within the leached layer of dissolving apatite; Incorporation mechanism of uranium by apatite
O) within the leached layer of dissolving apatite; Incorporation mechanism of uranium by apatiteOnuki, Toshihiko; Kozai, Naofumi; Samadfam, M.; Yasuda, Ryo; Yamamoto, Shunya; Narumi, Kazumasa; Naramoto, Hiroshi; Murakami, Takashi*
Chemical Geology, 211(1-2), p.1 - 14, 2004/11
Times Cited Count:48 Percentile:65.2(Geochemistry & Geophysics)no abstracts in English
Takahashi, Yoshio*; Yoshida, Hidekazu; Sato, Nana*; Hama, Katsuhiro; Yusa, Yasuhisa;
Chemical Geology, 184(3-4), p.311 - 335, 2002/00
Times Cited Count:137 Percentile:90.47(Geochemistry & Geophysics)None